The Zebrafish toxicology model
We can help you in assessing the toxicity and tolerance of your compounds.
Allows identification
- It allows the identification of drug actions and toxicity under conditions very similar to those in patients. Both general and specific toxicities in zebrafish correlate very well with toxicities in mice and humans.
Solves problems
- Solves problems with the fast metabolism of drugs in other animals (i.e., mice) by passive uptake of drugs from the water that results in control over the drug concentration in the embryo.
Provides data
- Provides data in a matter of days. Zebrafish embryos are widely used, faster, and less expensive than other in-vivo studies, and more precise than in-vitro experiments.
It is often difficult to translate toxic effects from cell culture to in-vivo toxicology. Here the zebrafish toxicology model fills a niche between in-vitro and in-vivo models.
General toxicology
General toxicology determines tissue-specific and non-specific toxicities from microscopic examination of the larvae over three days.
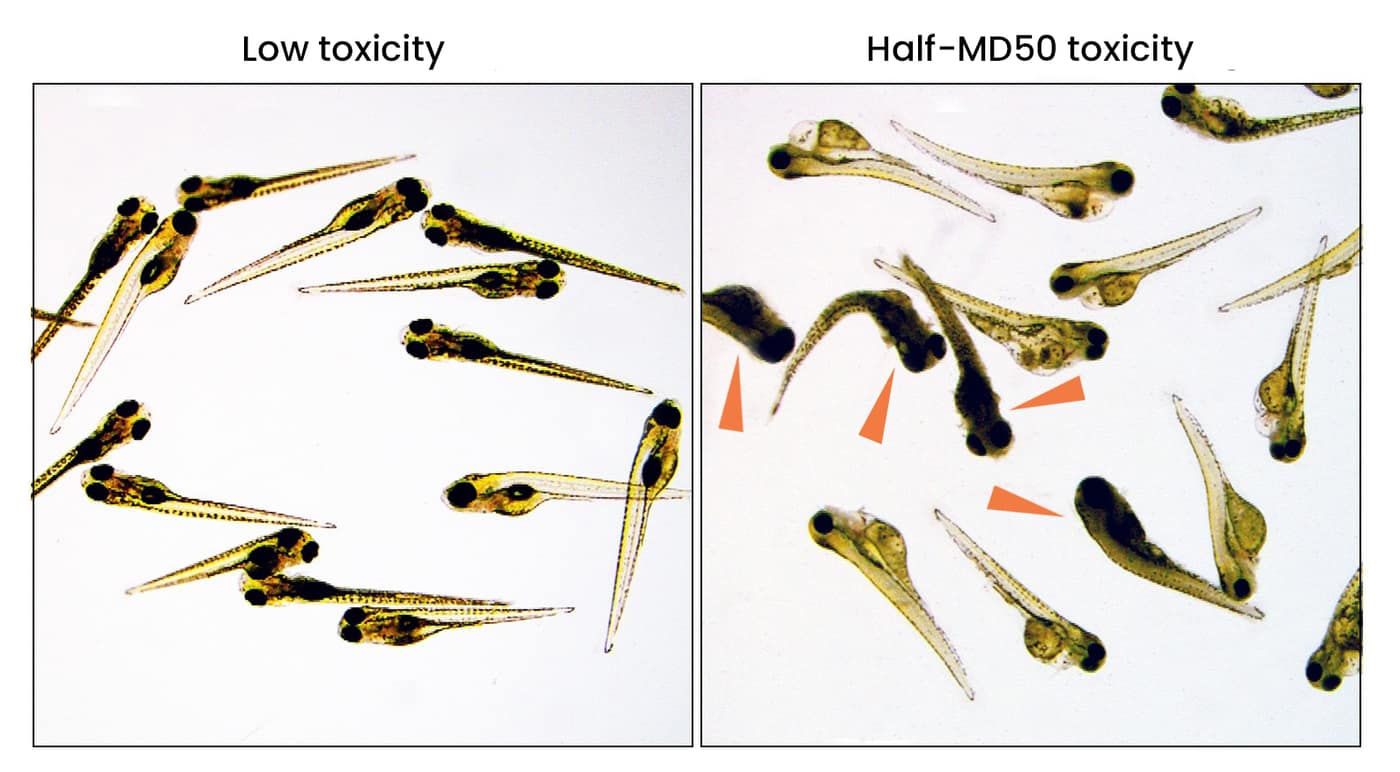
- Samples taken from the water and the zebrafish embryos over time can be analyzed for drug and drug metabolite concentrations to establish information related to the pharmacokinetics (administration, distribution, metabolism and elimination; ADME) of the drug.
- Determination of LD50 (50% lethal dose), MTD (maximum tolerated dose), NOAED (no observed adverse effects dose), and in-vivo pharmacokinetics are determined within days.
Specific toxicology
Specific toxicology determines organ-specific and non-specific toxicities from microscopic examination of the larvae over three days.
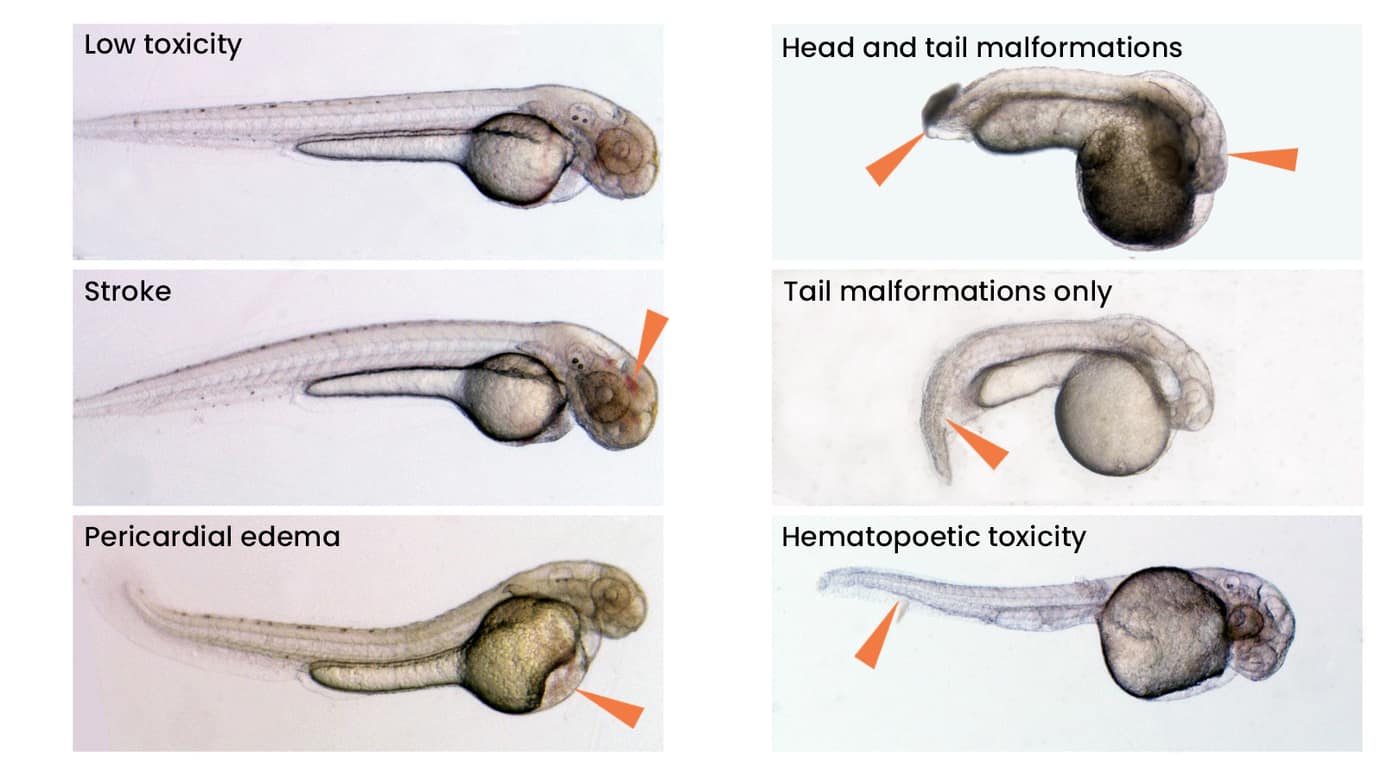
- Toxicology data for various organs (brain, liver, bone marrow, kidneys, cardiovascular system, endocrine glands, bone, muscle, GI tract, etc.) are collected through the use of transgenic reporter strains in which cells of a particular organ are labeled with a fluorescent protein.
