An agile and highly customizable toolbox for drug discovery
Assess the efficiency of new cancer treatments faster, cheaper, and more effective than current methods. Our platform streamlines evaluation, accelerating the development of safer and more potent therapies.


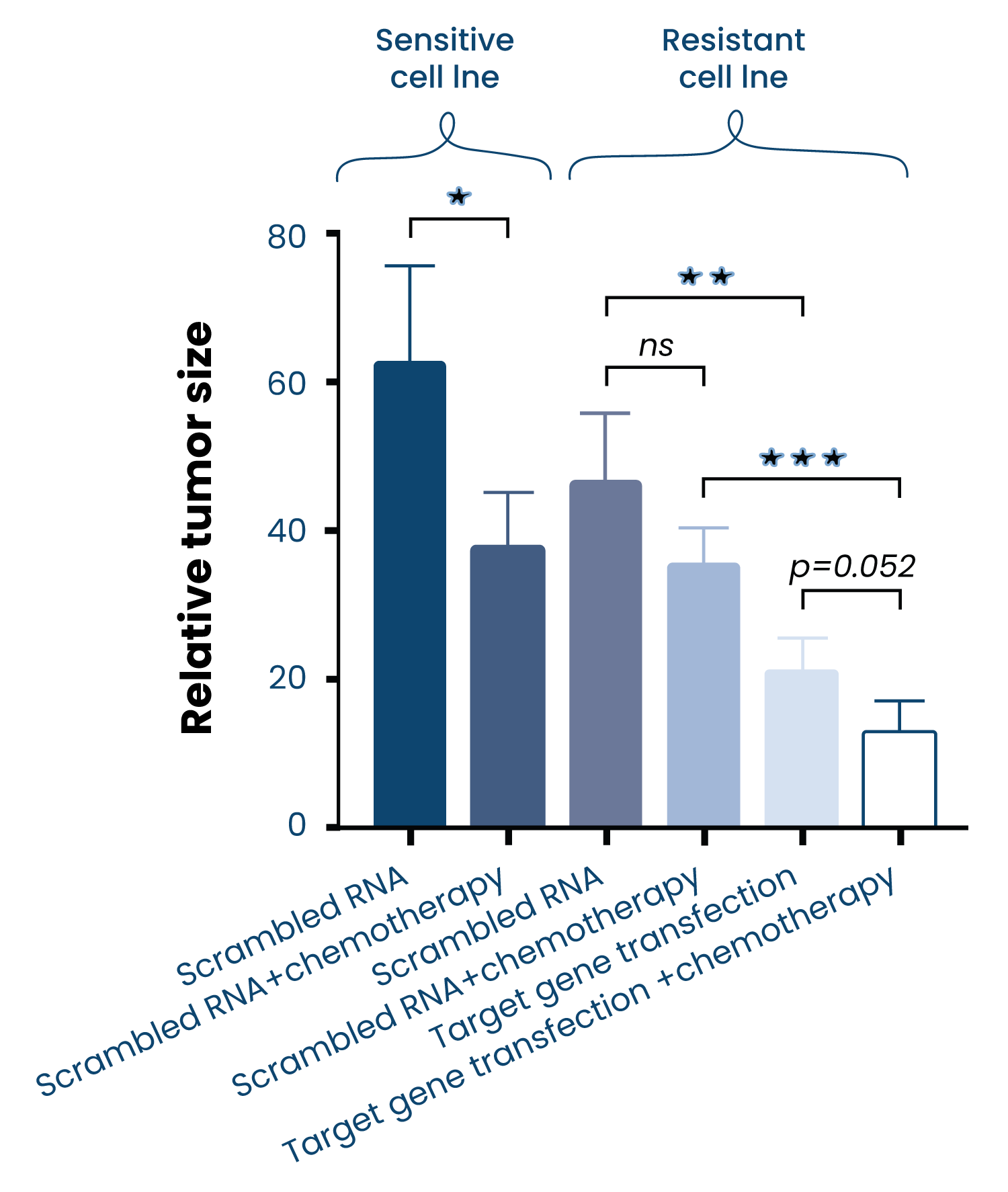
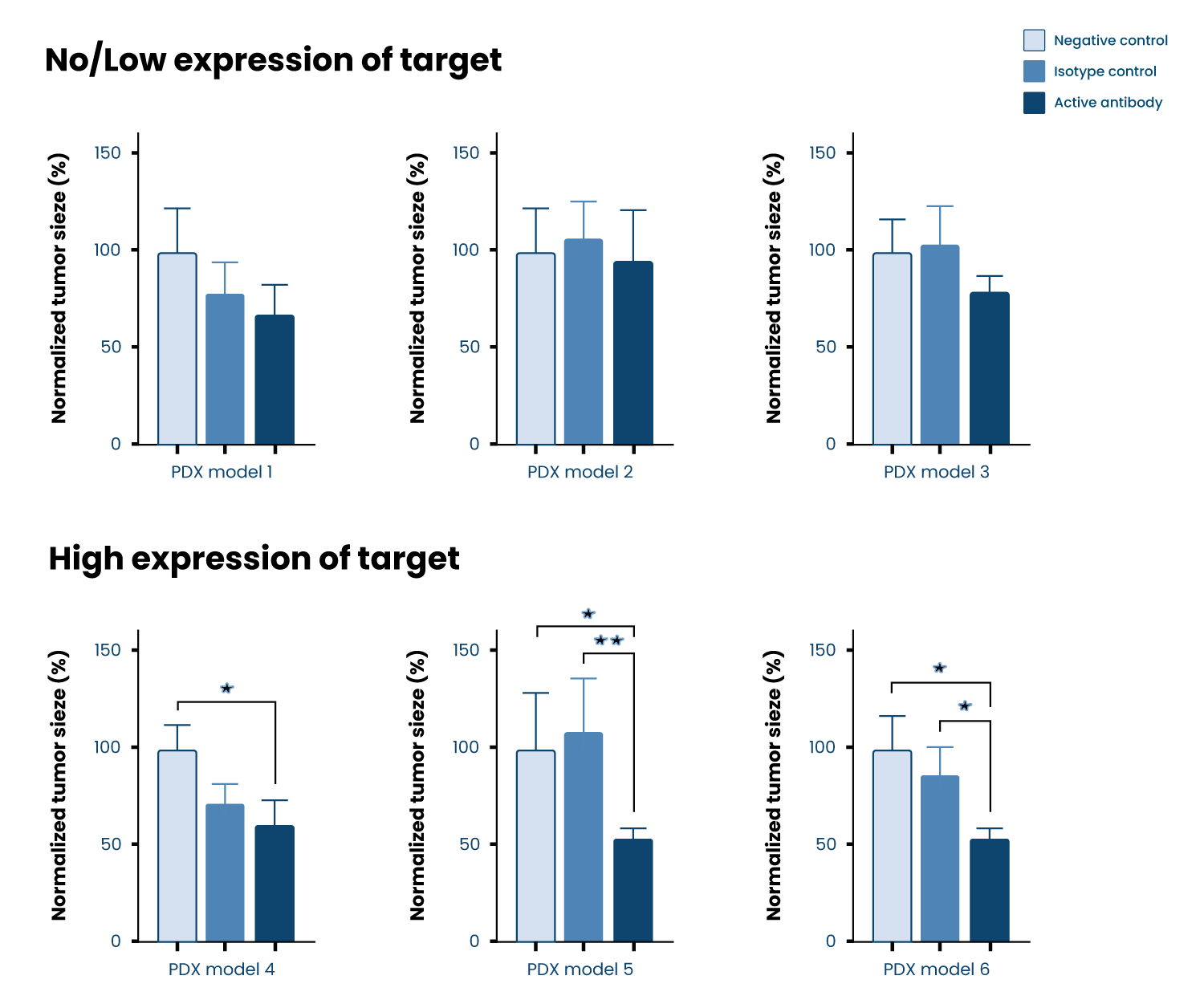
Our unique ZTX®platform is shortening time for drug discovery by providing in-vivo data on tumor regression and metastasis inhibition of compounds within 5 days
- Current overall tumor engraftment clinical samples: 89%
- Current overall tumor engraftment CRL models: 93%
- More than 40 cell lines in our in-house library
- Access to over 1000 fully characterized PDX models from our partner Charles River Laboratories and Xenostart
- Access to patient tumors for co-clinical trials
- Possibility to use any patient material or cell line

How it works
- A humanized tumor xenograft is created from fluorescent labeled patient-derived cell lines (CDX) or patient-derived xenograft (PDX) material in zebrafish larvae.
- The tumor microenvironment can be customized by co-implantation of stroma or immune cells to analyze cell-cell communication in the tumor.
- Tumor-bearing zebrafish larvae are treated with the lead drug candidates, such as small molecules, large molecules, antibodies, ADCs, or therapeutic cells, for three days. Treatments can be added to the water or injected.
- Output shows the efficacy of treatment-induced tumor regression and inhibition of metastasis invasion.
Reconstitute a patient specific tumor microenvironment with immune- and stromal cells
Gain new in-vivo insights into the mechanism of action of your drug through co-implantation of human tumor cells with non-malignant tumor-infiltrating cells to recapitulate the pathological communication between different cell types in the tumor microenvironment.

Our output
Tumor efficacy
- Fluorescent-labeled human cells can be visualized and followed over time in the transparent embryo.
- Tumor size is measured in the living embryo by fluorescent microscopy, assessing the tumor-killing capacity of the compounds being tested.
- Embryos are housed individually, and every embryo is tracked separately throughout the experiment.

Metastasis invasion
- The fast development of the embryo enables rapid vascularization and invasion of the developed microtumor.
- Metastatic cells will disseminate from the primary site into the vasculature and to the hematopoietic site in the embryo´s tail, where the metastatic cells will migrate to the tissue.
- The analysis includes the potential of the compounds being tested to inhibit both local and distal metastatic dissemination.